Acinar cell carcinoma of the pancreas: computed tomography features—a study of 15 patients
Siva P. Raman1 , Ralph H. Hruban2 , John L. Cameron3 , Christopher L. Wolfgang3 , Satomi Kawamoto1 and Elliot K. Fishman1
(1) | Department of Radiology, Johns Hopkins University School of Medicine, JHOC 3251, 601 N. Caroline Street, Baltimore, MD 21287, USA |
(2) | Johns Hopkins Medical Institutions, Johns Hopkins University School of Medicine, 401 North Broadway, Weinberg 2242, Baltimore, MD 21231-2410, USA |
(3) | Department of Surgery, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, Blalock Building, Room 679, 600 N. Wolfe Street, Baltimore, MD 21287, USA |
Published online: 18 February 2012
Abstract
Objective
Evaluation of the imaging features of pathology-proven acinar cell carcinomas (ACCs) of the pancreas using computed tomography (CT).
Methods
We reviewed the CT features, clinical presentations, and clinical outcomes of 15 patients (9 men, 6 women, mean age 62.3) with pathology-proven pancreatic ACCs. An abdominal radiologist retrospectively evaluated each patient’s initial imaging study with respect to the lesion’s size, location, attenuation (Hounsfield units) on arterial and venous phase images, peripancreatic lymphadenopathy, and distant metastases. Additional parameters studied included biliary and pancreatic ductal dilatation, intratumoral hemorrhage, calcification, the presence of cystic/necrotic components, and whether the tumor was intraparenchymal or exophytic.
Results
The ACCs in this series were evenly distributed between the head/uncinate and the tail, were predominantly exophytic (73%), tended to be large (average size 5.1 cm), and were mostly hypodense to the surrounding pancreas on both the arterial and venous phase images. A sizeable proportion demonstrated a cystic or necrotic component (53%) and/or an enhancing capsule (53%). Of those lesions in the head or uncinate process, very few resulted in pancreatic (28%) or biliary (14%) ductal dilatation. None of the lesions in this series showed internal calcification or intratumoral hemorrhage.
Conclusion
While a prospective diagnosis is difficult, ACCs have several features which can differentiate them from ductal adenocarcinoma, including their large size, lack of biliary or pancreatic ductal dilatation, exophytic nature, and the presence of an enhancing capsule.
Keywords Pancreas – Acinar cell carcinoma – Pancreatic ductal adenocarcinoma – Computed tomography
A rare neoplasm of the pancreas with differentiation that recapitulates acinar cells, acinar cell carcinoma (ACC) accounts for less than 1% of all pancreatic neoplasms [1]. The tumor is classically seen in older men, usually over the age of 50, who rarely can present with a unique syndrome characterized by skin rashes, polyarthragias, fevers, and fat necrosis (“lipase hypersecretion syndrome”) [1].
While ACC is a clearly distinct pathologic entity, it can be very difficult to prospectively differentiate these neoplasms from the more common pancreatic ductal adenocarcinomas (DACs) and pancreatic neuroendocrine tumors based on their CT imaging features. There have been very few comprehensive reviews of the imaging appearance of ACC, with the literature mostly limited to case reports and small case series. In this article, we present 15 patients at our institution with pathology-proven ACC, which we believe to be the largest series of its type in the radiology literature.
Materials and methods
Patients
A retrospective search of the pathology department database at our institution was conducted from 1985 to 2011, revealing 17 patients with pathology-proven ACC. Two cases were excluded from the study due to the unavailability of preoperative imaging. As a result, 15 patients were included in the study, all of whom had a contrast-enhanced CT as part of their preoperative evaluation.
Institutional review board (IRB) permission was obtained for review of the patients’ medical records, and patient informed consent was not required. The patients’ presenting symptoms, serum tumor markers, initial CT imaging report, pathology reports, and postoperative follow-up were reviewed (if available).
The 15 patients included in the study had a mean age of 62.3 years (range 47–77). Nine patients were men and 6 were women. Presenting symptoms included abdominal pain (n = 6), chronic anemia (n = 2), jaundice (n = 1), weight loss (n = 1), and multiple bouts of pancreatitis (n = 2). The tumor was an incidental imaging finding in 3 patients.
Of the 7 patients with an available preoperative lipase level, 4 had a markedly elevated lipase (1186-12,535 U/L), and 2 others had a mildly elevated lipase (89–484 U/L). Notably, none of these patients had any reported clinical symptoms suggestive of lipase hypersecretion syndrome. Three patients had a CA 19-9 level drawn prior to treatment, which was normal in all 3 patients.
All 15 patients underwent surgical resection of their tumor: 8 underwent a distal pancreatectomy with splenectomy, while 7 underwent either a classic Whipple or pylorus-sparing Whipple procedure.
CT imaging
All 15 patients in the study underwent a contrast-enhanced CT prior to surgery. An abdominal radiologist evaluated each case with respect to the following characteristics: (1) location in the pancreas, (2) whether the tumor was entirely within the pancreatic parenchyma or exophytic, (3) size of the tumor (maximum axial diameter), (4) presence of a cystic or necrotic component (and an estimate of the percentage of that cystic component), (5) presence of calcification, (6) presence of pancreatic ductal (>3 mm) or biliary ductal (>7 mm) obstruction, (7) enhancement of the mass relative to the normal pancreas on arterial and venous images using Hounsfield attenuation units, (8) presence of an enhancing capsule, (9) presence of distant metastatic disease on presentation (as determined by CT), (10) presence of peripancreatic lymphadenopathy on presentation, (11) presence of major venous or arterial vascular encasement (>180°), and (12) presence of intratumoral hemorrhage. In addition, we reviewed the radiology reports generated at the time of the initial CT, and noted the radiologist’s preoperative “most likely diagnosis”.
Results
The location of the tumors was divided among the pancreatic tail (n = 7), pancreatic head/uncinate process (n = 7), and pancreatic body (n = 1). 11 of the 15 tumors were classified as being partially or completely exophytic, while only 4 lesions were thought to be intraparenchymal (Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10). Including a single lesion in the pancreatic head which was too small to be visualized by CT (and was noted to be 1).
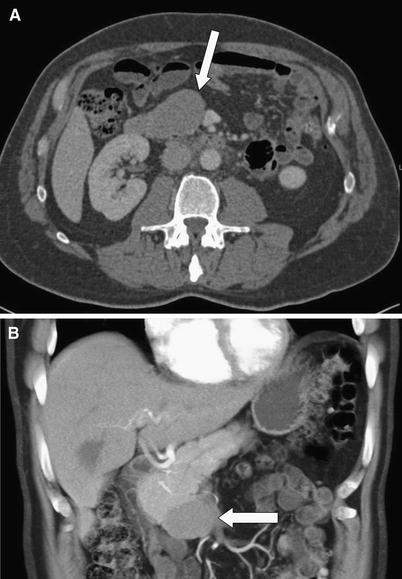
Fig. 1 A 71-year-old male with acinar cell carcinoma of the pancreatic uncinate process. Axial (A) and coronal volume rendered (B) images demonstrate a homogeneous, hypodense mass exophytically extending inferiorly from the uncinate process.

Fig. 2 A 55-year-old female with acinar cell carcinoma of the pancreatic head. Axial CT image demonstrates a cystic-solid mass (arrow) exophytically arising from the pancreatic head.

Fig. 3 A 56-year-old male with acinar cell carcinoma of the pancreatic tail. Axial CT images in the venous (A) and arterial (B) phases demonstrate a large, poorly defined, exophytic mass (arrows) extending from the pancreatic tail.

Fig. 4 A 47-year-old female with acinar cell carcinoma of the upstream pancreatic body/tail. Axial (A) and coronal volume rendered (B) CT images demonstrate a large well-circumscribed soft tissue mass (arrows) with mild internal cystic change.
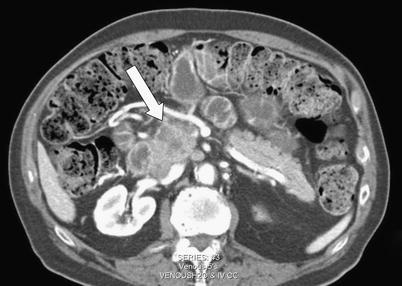
Fig. 5 A 77-year-old male with acinar cell carcinoma of the pancreatic uncinate process. Axial CT image demonstrates an exophytic soft tissue mass (arrows) with internal cystic change.
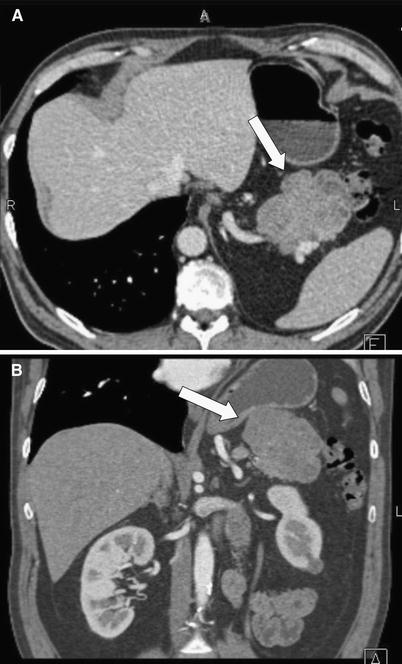
Fig. 6 A 56-year-old male with acinar cell carcinoma of the pancreatic tail. Axial (A) and coronal (B) CT images demonstrate a hypodense mass (arrows) arising from the pancreatic tail, with minimal internal cystic change.
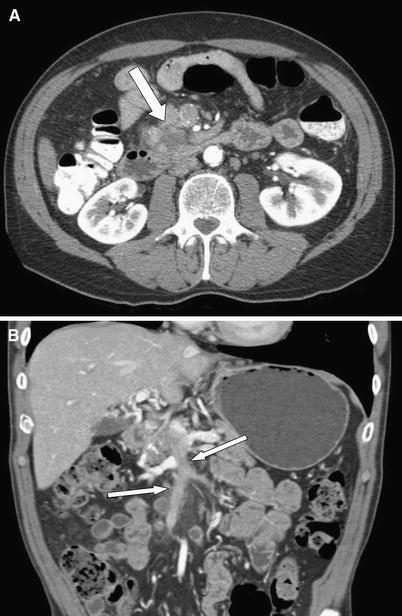
Fig. 7 A 65-year-old male with acinar cell carcinoma of the pancreatic uncinate process. Axial CT image (A) demonstrates a subtle hypodense mass (arrow) exophytically extending inferiorly from the pancreatic head/uncinate process. Coronal CT image (B) demonstrates enhancing tumor thrombus (arrows) extending throughout the main portal vein and SMV.

Fig. 8 A 70-year-old male with acinar cell carcinoma of the pancreatic tail. Axial (A) and coronal (B) CT images demonstrate a tubular, predominantly cystic mass (arrows) with enhancing mural nodules and an enhancing peripheral capsule.
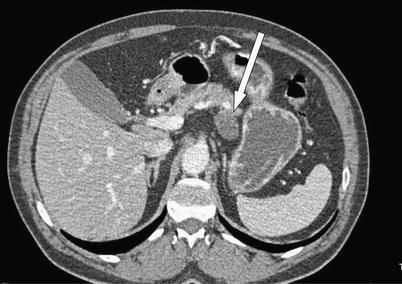
Fig. 9 A 67-year-old male with acinar cell carcinoma of the upstream pancreatic body/pancreatic tail. Axial CT images demonstrate a small, predominantly cystic mass extending exophytically from the pancreas.
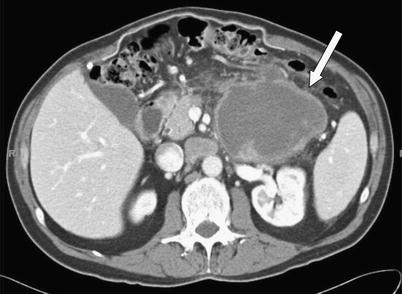
Fig. 10 A 60-year-old male with acinar cell carcinoma of the pancreatic tail. Axial contrast-enhanced CT image demonstrates a large, centrally necrotic mass (arrow) with peripheral, irregular enhancement.
Table 1 Imaging features of acinar cell carcinoma
CT features of 15 acinar cell carcinomas | Number of patients |
Exophytic component | 11 |
Purely intraparenchymal | 4 |
Cystic or necrotic | 8 |
<25% Cystic | 3 |
25–75% Cystic | 2 |
>75% | 3 |
Internal calcification | 0 |
Intratumoral hemorrhage | 0 |
Enhancing peripheral capsule | 8 |
Eight of the 15 (53%) tumors had a cystic or necrotic component (Figs. 2, 4, 5, 6, 7, 8, 9, 10). Of these, 2 lesions showed greater than 75% necrosis/cystic change, 2 between 50 and 75%, and 3 under 25%. None of the lesions demonstrated any intratumoral calcification or hemorrhage. Of the 7 lesions in the pancreatic head or uncinate process, only 1 showed biliary ductal dilatation (14%) and only 2 (28%) showed significant pancreatic ductal dilatation.
Nine of the 15 lesions were clearly hypovascular to the surrounding pancreas on the arterial phase images (at least 10 HU below the surrounding pancreas), 1 was mildly hypervascular (10 HU or greater compared to the surrounding pancreas), and the remainder were essentially isodense (within 10 HU). On the venous phase images, 12 lesions were clearly hypodense, 3 were essentially isodense, and none were hypervascular. 8 of the 15 lesions had a clearly demarcated enhancing capsule on either the arterial or venous phase images.
Only 1 of the 15 patients had identifiable distant metastatic disease on their initial CT, and 5 had significant peripancreatic lymphadenopathy (>1 cm in short axis). 3 patients demonstrated vascular narrowing/occlusion of the splenic vein or splenic artery, and 1 patient had frank enhancing tumor thrombus within the portal vein and SMV (Fig. 7). Nine of the 15 patients had at least one malignant peripancreatic lymph node identified at surgery.
A review of the “most likely” preoperative diagnosis provided by the radiology service included DAC (n = 4), neuroendocrine tumor (n = 8), cystic pancreatic tumor/IPMN (n = 4), and lymphoma (n = 1). The diagnosis of ACC was not made prospectively in any of the cases.
While only a single patient demonstrated distant metastatic disease on their initial CT, 7 other patients ultimately developed metastatic disease during the course of their follow-up.
A review of the clinical records revealed that 2 patients died during the course of their follow-up at our institution, having lived 975 and 1148 days, respectively (average 1061.5 days/35.3 months). Two patients were lost to follow-up immediately after surgery, and their post-operative treatment and course were not available.
Five patients are still alive at the time of the writing of this article, having lived between 252 and 1653 days (average 665 days/22.1 months, median 525 days/17.5 months). The remaining patients in the study were lost to follow-up at between 138 and 1227 days (average 749.7 days/24.9 months, median 811.5 days/27.1 months). Three of these 6 patients were lost to follow-up when disease free at between 543 and 949 days (average 722 days/24 months).
Discussion
Although >80% of the pancreas is composed of acinar cells, and only 4% is composed of ductal epithelial cells, ACC is significantly rarer than pancreatic DAC, representing <1% of all pancreatic neoplasms [1, 2]. Given the rarity of the tumor, there are very few large series of these tumors in either the clinical or radiologic literature, and much of our knowledge of the imaging characteristics of ACC is based on case reports and small series. To the best of our knowledge, we believe our series of 15 patients to be the largest of its kind in the radiology literature.
Traditionally, ACC has been considered a carcinoma of older adults, with a strong male predilection [3, 4]. The mean age of the patients in our series was 62, similar to a number of other series where the mean age was >60, and there was a mild male predilection (60%). With the exception of a single patient who presented because of jaundice and biliary ductal obstruction, the initial presentation of the vast majority of these patients was nonspecific. Presenting symptoms included abdominal pain, weight loss, chronic anemia, and repetitive bouts of pancreatitis, with abdominal pain being the most common presentation (6 of 15 patients).
Notably, while ACC has often been popularly associated with the “lipase hypersecretion syndrome,” a syndrome characterized by fevers, arthralgias, skin rashes, and fat necrosis as a result of the tumor’s secretion of lipase into the blood stream, none of the patients in this series showed clinical manifestations of this syndrome at the time of diagnosis. Interestingly, despite this, 4 patients had markedly elevated lipase levels prior to surgery (>1186 U/L), while another 2 patients had mildly elevated lipase levels (5]. No consistently elevated tumor markers were identified in any of these patients: As in other series, CA 19-9 levels were normal in 3 patients [3, 6].
While other series have suggested that these neoplasms have a propensity for the pancreatic head, the tumors in this series were fairly evenly divided between the head/uncinate process (7/15) and the pancreatic tail (7/15), with only a single lesion in the central pancreatic body (1/15) [2]. The single most characteristic feature of the lesions in our series was their exophytic nature on imaging: 11 of the 15 tumors either partially, or completely, extended from the pancreatic parenchyma. Many of the lesions were well circumscribed, and 8 lesions had an enhancing capsule, usually visualized on the venous phase images. A sizeable number of lesions showed significant cystic or necrotic change (8/15), and 5 of these showed >50% cystic component. This is similar to findings reported by Tatli et al. [5], a series in which up to 55% had cystic components. Notably, while intratumoral calcification has been described as a common feature of ACC, none of the lesions in this series had any calcification [5].
Of those tumors in the pancreatic head or uncinate, only 1/7 (14%) showed any biliary ductal dilatation, and only 2/7 (28%) showed pancreatic ductal dilatation. This feature of ACC has been previously described, and is thought to reflect the origin of these lesions from the acinar cells of the pancreas, rather than the ductal epithelium [4]. As a result, unlike DAC, very few patients with ACC present with painless jaundice, a finding confirmed in our series of patients [6].
Reviewing these cases, although the vast majority of these tumors were hypodense on the arterial and venous phase images relative to the normal pancreatic parenchyma, several features retrospectively made the diagnosis of DAC less likely: (1) large size, with a mean maximum diameter of 5.1 cm (as opposed to a mean size of 2-3 cm for DAC) [4]; (2) exophytic nature of the tumor; (3) the presence of a well-defined enhancing capsule (53%); (4) cystic or necrotic change (53%); (5) the lack of significant biliary or pancreatic ductal dilatation, despite the presence of a fairly large mass at the pancreatic head or uncinate process; and (6) a relative lack of vascular encasement or narrowing (20%). Accordingly, these lesions were mistaken for DAC prospectively by the radiologist in only 4 of the 15 cases. It was more common to mistake these lesions for a neuroendocrine tumor, or in those cases where the cystic component predominated, for an IPMN or other cystic pancreatic neoplasm. Notably, however, although these tumors were often mistaken for neuroendocrine tumors, only 1 of the 15 lesions could be classified as hypervascular.
Ultimately, while the focus of this study was on the imaging features of ACC, rather than its clinical course, the aggressive nature of the lesion was clear in the available follow-up. While only 1 patient demonstrated distant metastatic disease on their initial CT, 7 others ultimately developed metastatic disease during their follow-up at our institution. While only 5 patients had evidence of significant peripancreatic lymphadenopathy by CT criteria, 9 patients were found to have malignant lymphadenopathy at surgery. Nevertheless, ACC is considered to have a slightly better prognosis than DAC, and our results seem to support this [7]: Of those patient still being followed, 5 are currently alive, having lived an average of 22.1 months, and of those patients lost to follow-up, 3 were disease free at an average of 24 months. The most common site of metastatic disease was the liver (6 patients), with other sites including the lung (2 patients), carcinomatosis (4 patients), bone (1 patient), or recurrence in the surgical bed (2 patients). Notably, the rate of distant metastatic disease at presentation in our series (7%) was lower than in some other series, where rates up to 50% have been reported [2, 8].
In conclusion, the imaging diagnosis of ACC is extremely difficult to make prospectively. In fact, the diagnosis of ACC was not considered in the initial radiology reports for any of the 15 patients in our series, while a number of other diagnoses were considered, including DAC, neuroendocrine tumor, lymphoma, and cystic pancreatic neoplasm. However, the lesions in this study had several features in common, which we believe to be suggestive: When confronted with a large, exophytic, well-circumscribed mass arising from the pancreas, often without appreciable biliary or pancreatic ductal dilatation, the diagnosis of ACC should be considered.
References
1. | Chiou Y, Chaing J, Hwang J, et al. (2004) Acinar cell carcinoma of the pancreas: clinical and computed tomography manifestations. J Comput Assist Tomogr 28(2):180–186 |
2. | Khalili M, Wax BN, Reed WP, et al. (2006) Acinar cell carcinoma of the pancreas. Clin Imaging 30:343–346 |
3. | Hartwig W, Denneberg M, Bergmann F, et al. (2011) Acinar cell carcinoma of the pancreas: is resection justified even in limited metastatic disease. Am J Surg 202:23–27 |
4. | Hsu M, Pan K, Chu S, et al. (2010) CT and MRI features of acinar cell carcinoma of the pancreas with pathological correlations. Clin Radiol 65:223–229 |
5. | Tatli S, Mortele KJ, Levy AD, et al. (2005) CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol 184:511–519 |
6. | Matos J, Schmist C, Turrini O, et al. (2009) Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg 13:1495–1502 |
7. | Wisnoski NC, Townsend CM, Nealon WH, et al. (2008) 672 patients with acinar cell carcinoma of the pancreas: a population based comparison to pancreatic adenocarcinoma. Surgery 144(2):141–148 |
8. | Gravante G, Williams RN, Dennison AR, Bowrey DJ (2011) Pancreatic acinar cell carcinoma. Surgery. doi:10.1016/j.surg.2011.07.024 |
